What are Subatomic Particles?
For a long time, it was believed that atoms are the ultimate particles that matter is made up of and that these atoms cannot be divided further. The experiments conducted during the latter half of the nineteenth century and early years of the twentieth century revealed that the atom is not the ultimate particle. The continued efforts of the scientists led to the discovery of subatomic particles.
The three primary subatomic particles that constitute an atom are illustrated below.
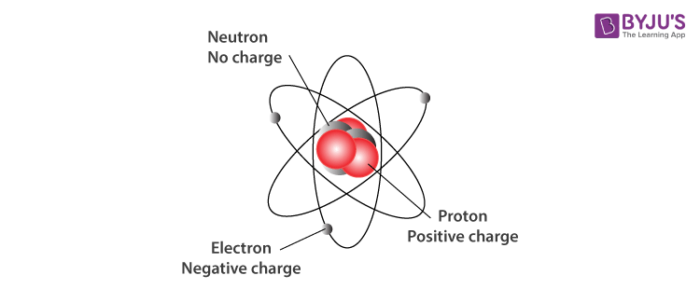
The limitations of Dalton’s atomic theory to explain certain observations formed the basis for the discovery of electrons and protons. Further investigations revealed the existence of neutrons. The components of atoms are called subatomic particles and generally include the proton, electron, and neutron.
Discovery and Features of Subatomic Particles
The discovery of the three basic subatomic particles and some of their important features are discussed in this subsection.
Protons
Protons and Neutrons together make up the nucleus of an atom and hence are called nucleons. Some important points regarding the discovery and properties of protons are listed below.
- Protons are positively charged subatomic particles.
- The number of protons in an atom is equal to the number of electrons in it.
- The discovery of protons is credited to Ernest Rutherford.
- Protons can be produced via the removal of an electron from a hydrogen atom.
- The mass of a proton is 1.676 * 10-24 grams.
- The charge of a proton is +1.602 * 10-19 Coulombs.
Click here for a detailed article about protons.
Electrons
Electrons are subatomic particles that revolve around the nucleus of an atom. Ions can be formed either by the loss or gain of electrons. Electrons of different atoms come together to participate in chemical bonding. A few points detailing the discovery and the properties of electrons are listed below.
- Electrons are negatively charged subatomic particles.
- An equal number of electrons and protons are found in the atoms of all elements.
- J. J. Thomson is credited with the discovery of electrons since he was the first person to accurately calculate the mass and the charge of an electron.
- The mass of an electron is negligible when compared to the mass of a proton. It is found to have a mass equal to (1/1837) times the mass of a proton.
- The charge of an electron is equal to -1.602 * 10-19 Coulombs.
Click here to learn more about electrons.
Neutrons
Neutrons, along with protons, make up the nucleons. Neutrons are named for their neutral nature – unlike protons and electrons, they do not carry any charge. The discovery and general properties of neutrons are discussed below.
- Neutrons are neutrally charged subatomic particles.
- The masses of two different isotopes of an element vary due to the difference in the number of neutrons in their respective nuclei.
- The neutron was discovered by James Chadwick in 1932.
- They were discovered in an experiment wherein a thin sheet of beryllium was bombarded with alpha particles.
- The mass of a neutron is 1.676 * 10-24 grams.
To learn more about neutrons, click here.
Thus, the discovery and the general properties of the three primary subatomic particles are covered. However, advances in atomic structure and quantum mechanics have led to the discovery of other fundamental particles. The discovery of subatomic particles has been the base for many other discoveries and inventions.
Frequently Asked Questions – FAQs
What are the three types of subatomic particles?
Subatomic particles include electrons, negatively charged, nearly massless particles that account for much of the atom’s bulk, that include the stronger building blocks of the atom’s compact yet very dense nucleus, the protons that are positively charged, and the strong neutrons that are electrically neutral.
Which is the smallest subatomic particle?
Quarks represent the smallest subatomic particle.
Is anything smaller than an atom?
Subatomic particles are lighter than atoms in the physical sciences. They may be artificial particles, such as neutrons and protons, or elementary particles that are not constructed of such particles in compliance with the standard model.
Is a photon smaller than an atom?
The Quantum of Electromagnetic Radiation is a photon, while an atom is the central part of all matter. Its size can be close to the size of Electron-like subatomic particles, but it is smaller than an atom.
Does a photon have a size?
If “size” means mass or diameter a photon is said to have neither.
