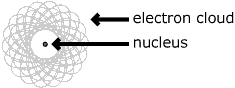
| The atom consists of a tiny nucleus surrounded by moving electrons. The nucleus contains protons, which have a positive charge equal in magnitude to the electron's negative charge. The nucleus may also contain neutrons, which have virtually the same mass but no charge. | |||
|
Often the atom is described like a sphere with the nucleus in the center and the electrons encircling the nucleus. The electrons take up most of the atomic space, but only account for a tiny proportion of the atom's mass. A simple view of a nuclear atom is shown below. It is the differences in the number and arrangement of the electrons that cause atoms to have different chemical properties. | ||