Stereochemistry is the branch of chemistry that involves “the study of the different spatial arrangements of atoms in molecules”.
Stereochemistry is the systematic presentation of a specific field of science and technology that traditionally requires a short preliminary excursion into history. Stereochemistry is the ‘chemistry of space ‘, that is stereochemistry deals with the spatial arrangements of atoms and groups in a molecule.
Stereochemistry can trace its roots to the year 1842 when the French chemist Louis Pasteur made an observation that the salts of tartaric acid collected from a wine production vessel have the ability to rotate plane-polarized light, whereas the same salts from different sources did not have this ability. This phenomenon is explained by optical isomerism.
Table of Contents
- Facts about Stereochemistry
- Types of Stereoisomers
- Stereoisomerism
- Recommended Videos
- Importance of Stereochemistry – Thalidomide Disaster
- Frequently Asked Questions – FAQs
Facts about Stereochemistry
- The structure of a molecule can vary based on the three-dimensional arrangement of the atoms that constitute it. Stereochemistry also deals with the manipulation of the arrangement of these atoms.
- This branch of chemistry is commonly referred to as 3-D chemistry since it focuses on stereoisomers (chemical compounds with the same chemical formula but a different spatial arrangement in three dimensions).
- One of the branches of stereochemistry deals with the study of molecules that exhibit chirality, which is a geometric property of molecules that makes them non-superimposable on their mirror images.
- Another branch of 3-D chemistry, known as dynamic stereochemistry, involves the study of the effects of different spatial arrangements of atoms in a molecule on the rate of a chemical reaction.
Types of Stereoisomers
| Atropisomerism | Atropisomers are stereoisomers resulting from hindered rotation about one or more single bonds. This is observed in case of many drugs. |
| Cis-trans isomerism | Cis-trans isomerism shares the same atoms which are joined to one another in the same way but have a different configuration. This is generally observed in the case of alkenes and complexes. |
| Conformational isomerism | Conformational isomerism is a type of stereoisomerism in which isomers can only be converted by formally single bond rotations. This is observed in single-bonded systems like alkanes. |
| Diastereomers | Diastereomers are optically active isomers that are not enantiomers. |
| Enantiomers | An enantiomer is one of a pair of optical isomers, the structures of which are not superimposable on their mirror images. Chirality becomes the criteria here. |
Stereoisomerism
Stereoisomerism refers to “the isomerism that is caused by the non-similar arrangements of atoms or functional groups belonging to an atom in space”. These types of isomers have the same constitutions, but different geometric arrangements of atoms. Stereoisomers can be broadly classified into two types, namely enantiomers and diastereomers.
1. Enantiomers
- When two isomers are mirror images of each other, the type of isomerism is called enantiomerism and these isomers are referred to as enantiomers.
- Enantiomers are stable and isolable compounds that differ in their spatial arrangements in 3-D space.
- They generally exist as discrete pairs.
- The properties of enantiomers are identical. However, their interaction with a plane of polarized light can vary.
- The direction in which they rotate the plane-polarized light is different, that is, if one rotates in the right direction, the other rotates towards the left.

2. Diastereomers
- When two isomers do not behave as mirror images of each other, they are called diastereomers.
- A molecule with ‘n’ number of asymmetric carbon atoms can have up to ‘2n’ diastereomers.
- When two diastereomers differ at only one stereocenter, they are referred to as epimers.
- These isomers vary in physical properties and chemical reactivity.
An illustration of enantiomers that are mirror images of each other is provided below.
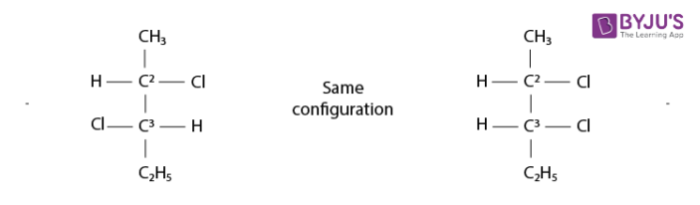
Enantiomers in Stereochemistry – Mirror Image