Sodium bicarbonate or Sodium hydrogen carbonate has a monoclinic crystalline structure. Nicolas Leblanc a French chemist produced sodium carbonate in the year 1791. In the year 1846 Austin Church and John Dwight, bakers of New York started the first factory to produce baking soda. It is a white solid crystalline chemical compound usually in its powder form. This salt is composed of sodium ions and bicarbonate ions. Its molecular formula is NaHCO3. It is a weak base. It is commonly called as baking soda and is used in cooking. PH value is about 8.31.
Properties of Sodium Bicarbonate
| Sodium bicarbonate Chemical formula | NaHCO3 |
| Molecular Weight/ Molar Mass | 84.0066 g/ mol |
| Density | Solids: 2.20 g/cm3 Powder: 1.1 – 1.3 |
| Boiling Point | 851 °C |
| Melting Point | 50 °C |
A few other important properties of sodium hydrogen carbonate are listed below.
- NaHCO3 has a white, crystalline appearance.
- This compound is insoluble in ethanol and slightly soluble in methanol and acetone.
- At a temperature of 20 degrees celsius, the solubility of this compound in water corresponds to 96 grams per litre.
- Sodium bicarbonate crystallizes in a monoclinic crystal lattice.
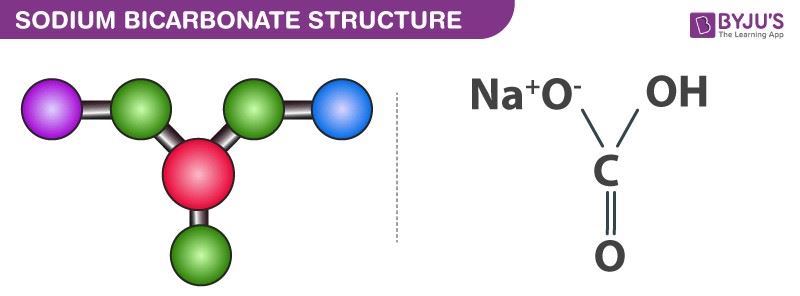
Sodium Bicarbonate Structure (NaHCO3)
Sodium bicarbonate molecules feature one sodium cation and one bicarbonate anion. Here, an ionic bond is formed between the positively charged sodium ion and the negatively charged oxygen (which is singly bonded to the central carbon and not bonded to a hydrogen atom).
Uses of Sodium Bicarbonate
- It is used as pest control to kill cockroaches and controlling fungal growth
- It is used as a disinfectant
- It is used to protect armpits from bad smell and irritation
- It is used in cooking especially to bake food items
- It is used in medicine to be injected intravenously to the prevention of chemotherapy side effects
- It is used to wash kitchen products due to its antibacterial properties
- It is used to have clean teeth and mouth